Обновлено: 30 May 2022.
Refrigerant R134a (tetrafluoroethane) is a colorless gas with a boiling point of -29 °C. Due to its technical characteristics, r134a refrigerant is used in air conditioners, chillers, refrigeration equipment, polymer production, medicine, and cosmetology. He is known as:
- HFC R134a;
- Freon-134;
- Refrigerant R134-a;
- Refrigerator 134a;
- Freon™ 134a;
- HFC 134a.
We will talk about the characteristics of refrigerant-134, physical and chemical properties, application. You will learn about the features of the use and operation of equipment on the R134a. Here are tables of boiling point, refrigerant pressure r134-a, properties of saturated vapor and liquid under different conditions.
History of appearance
In 1987, the Montreal Protocol on Substances that Deplete the Ozone Layer was signed, according to which ozone-depleting refrigerants were gradually withdrawn from use and use. R134a (HFC 134a) is a gas designed to replace R12. It has zero ozone depletion potential.
Refrigerant-134 has gained popularity due to its low cost and global warming potential. It affects the greenhouse effect 8.39 times less than refrigerant R12. But when replacing it, it must be disposed of.
Technical characteristics of freon r134a
| Characteristic R134a | Meaning |
|---|---|
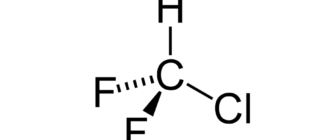
| chemical name | 1,1,1,2-Tetrafluoroethane |
| Chemical formula | CH2FCF3 |
| Molecular weight | 102.03 |
| Boiling point at one atmosphere | -26.06°C |
| Critical temperature | 101.08°C |
| critical pressure | 4060.3 kPa |
| Critical Density | 515.3 kg/m³ |
| Critical volume | 0.00194 m³/kg |
| Freezing point (°C) | -103 |
| Liquid density at 25°C (kg/m³) | 1.206 |
| Liquid density at 0°C (kg/m³) | 1.293 |
| Density of saturated vapor at the boiling point | 5.28 kg/m³ |
| Steam pressure (25°C) at 1 bar | 6.657 |
| Steam pressure (0°C) at 1 bar | 2.92 |
| Heat of vaporization at boiling point | 217.2 kJ/kg |
| Specific heat capacity of liquid at 25°C and 1 atm | 1.44 kJ/kg |
| Specific heat capacity of a liquid at 0°C and 1 atm | 0.85 kJ/kg |
| Liquid viscosity at 25°C (Pa s) | 0.202 |
| Steam viscosity at 25°C (Pa s) | 0.012 |
| Surface pressure at 25°C (mN/m) | 8.09 |
| Solubility in water at 25°C and 1.013 bar (wt%) | 0.15 |
| Solubility of water in R134a at 25°C and 1.013 bar | 0.11 (wt%) |
| Volumetric cooling capacity at -25°C | 1192.11 c/m³ |
| Heat capacity of steam at 25°C and 1 atm (kJ/kg K) | 0.852 |
| Thermal conductivity of liquid at 25°C (W/m K) | 0.0824 |
| Thermal conductivity of steam at 25°C (W/m K) | 0.0145 |
| Flammability | Not |
| ODP (Ozone Depletion Potential) | 0 |
| GWP (Global Warming Potential) | 1430 |
| Smell | Weak, sweet |
Application, use
R134a is a one-component gas. In the event of a leak, it does not require a full refill. Equipment operating on this refrigerant can be recharged without loss of efficiency. This is a big advantage compared to air conditioning and cooling systems based on R410a, R407c, R404. Also, R134a gas is used as a component of all effective substitutes for R22.
Refrigerant R134a is non-toxic, it is used in the production of inhalers, aerosols, capsules for freezing injuries. Subject to the elementary rules of safety and use, a negative impact on human health can be avoided.
Tetrafluoroethane is non-flammable and non-explosive under normal conditions. It has no concentration limits for flame propagation. Therefore, it is used as a propellant, a foaming agent for the production of foams.
PROPELLENTS are inert chemicals that create excess pressure in aerosol cans to force the active compound out of the package and disperse it in the environment.
The molar mass of tetrafluoroethane is 102.03 g/mol, air is 29 g/mol. In the event of a leak, the gas descends and accumulates at floor level, in the basement. This property of HFC-134a prevents it from entering the lungs under normal conditions.
Refrigerant-134a is not compatible with mineral oils. When they come into contact, foaming, oils entering the system, and deposition on its walls are possible. This leads to the formation of constricted places and blockages. Equipment using this refrigerant must be filled with PAG or POE synthetic oil.
The R134a molecule is smaller than the R12 molecule. Accordingly, it can penetrate into cracks through which R-12 refrigerant cannot escape. Therefore, HFC-134a climate equipment is more sensitive to mechanical damage.
At the moment, this gas is not banned, but everything can change. Every year, more stringent requirements are imposed on refrigerants. Due to its strong greenhouse effect, its use may be restricted. Therefore, analogues and substitutes for freon R134a are being actively developed.
Now, air conditioners and refrigerators on r134a are rare. They are being replaced by isobutane (r600a) technology. This refrigerant is cheaper, although it has a lower cooling capacity. Many specialists fill instead of the 134th refrigerant, 600th. But calculation of the required amount can only be based on experience. We described in more detail about the difference between these freons in an article about comparing refrigerants R-134a and R-600a.
Temperature, pressure, density, surface tension R134a
This table lists the following values:
- P is the pressure in kPa;
- ρ is the density in kg/m3;
- σ is the surface tension in mN/m.
| t, ℃ | P pair | ρ liquid | ρ couple | σ |
|---|---|---|---|---|
| -70 | 0.077 | 1453 | 0.47 | 23.1 |
| -60 | 0.155 | 1424 | 0.902 | 21.4 |
| -fifty | 0.289 | 1395 | 1.615 | 19.8 |
| -40 | 0.506 | 1366 | 2.727 | 18.2 |
| -thirty | 0.838 | 1336 | 4.382 | 16.6 |
| -20 | 1.325 | 1306 | 6.745 | 15.1 |
| -ten | 2.01 | 1276 | 10.01 | 13.6 |
| 0 | 2.939 | 1244 | 14.41 | 12.1 |
| ten | 4.165 | 1211 | 20.21 | 10.7 |
| 20 | 5.741 | 1178 | 27.74 | 9.24 |
| thirty | 7.725 | 1142 | 1142 | 7.86 |
| 40 | 10.18 | 1105 | 49.8 | 6.52 |
| fifty | 13.17 | 1064 | 65.69 | 5.22 |
| 60 | 16.77 | 1020 | 86.33 | 3.98 |
| 70 | 21.08 | 969.8 | 113.8 | 2.81 |
| 80 | 26.19 | 909.6 | 152.5 | 1.75 |
| 90 | 32.25 | 828.8 | 213.5 | 0.83 |
| 100 | 39.42 | 720.1 | 378 | 0.1 |
Table of pressure, boiling point of freon r134a (p-t char)
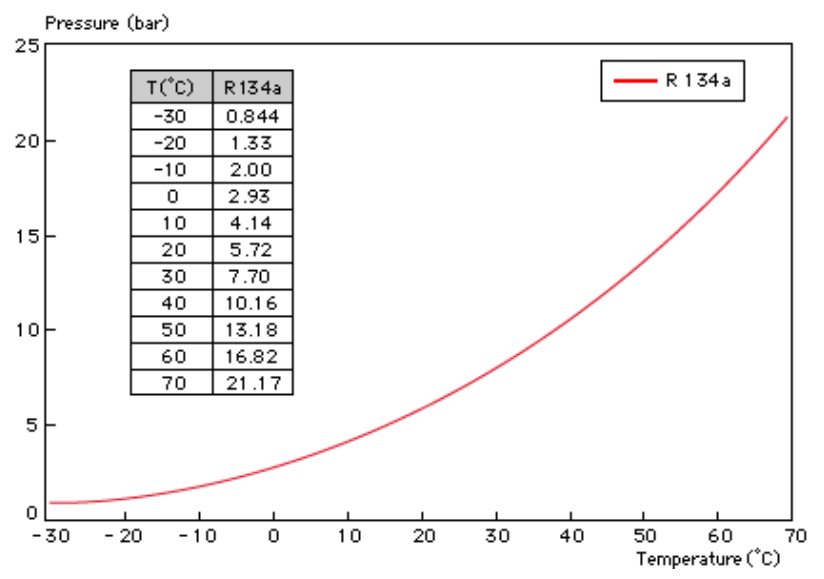
Below is a refrigerant R134a p-t chart:
| t, °C | P, bar(s) | t, °C | P, bar(s) | t, °C | P, bar(s) |
|---|---|---|---|---|---|
| -70 | -0.93 | -22 | 0.20 | +26 | 5.84 |
| -68 | -0.92 | -20 | 0.31 | +28 | 6.26 |
| -66 | -0.91 | -18 | 0.43 | +30 | 6.69 |
| -64 | -0.89 | -16 | 0.56 | +32 | 7.14 |
| -62 | -0.87 | -14 | 0.70 | +34 | 7.61 |
| -60 | -0.85 | -12 | 0.84 | +36 | 8.11 |
| -58 | -0.83 | -10 | 0.99 | +38 | 8.62 |
| -56 | -0.81 | -8 | 1.16 | +40 | 9.15 |
| -54 | -0.78 | -6 | 1.33 | +42 | 9.71 |
| -52 | -0.75 | -4 | 1.51 | +44 | 10.29 |
| -fifty | -0.72 | -2 | 1.71 | +46 | 10.89 |
| -48 | -0.68 | 0 | 1.92 | +48 | 11.52 |
| -46 | -0.64 | +2 | 2.13 | +50 | 12.17 |
| -44 | -0.60 | +4 | 2.36 | +52 | 12.84 |
| -42 | -0.55 | +6 | 2.61 | +54 | 13.54 |
| -40 | -0.50 | +8 | 2.86 | +56 | 14.27 |
| -38 | -0.44 | +10 | 3.13 | +58 | 15.02 |
| -36 | -0.38 | +12 | 3.42 | +60 | 15.81 |
| -34 | -0.32 | +14 | 3.72 | +62 | 16.62 |
| -32 | -0.25 | +16 | 4.03 | +64 | 17.45 |
| -thirty | -0.17 | +18 | 4.36 | +66 | 18.32 |
| -28 | -0.09 | +20 | 4.70 | +68 | 19.22 |
| -26 | 0.00 | +22 | 5.07 | +70 | 20.16 |
| -24 | 0.10 | +24 | 5.44 | – | – |
Material Compatibility
R134a is compatible with a number of sealing materials. It does not react with seals made from the following substances:
- ALATHON;
- Polypropylene;
- Chlorinated PVC;
- Ionomer resin;
- Epoxy resins;
- Polyacytamine;
- Polycarbonate;
- Polybutylene terephthalate;
- Polyarylate;
- Polyamide;
- Polysulfide.
R-134a may degrade itself or destroy system components upon contact. This refrigerant may react with the following seal materials:
- Polystyrene (Styrene);
- Polytetrafluoroethylene (PTFE);
- ETFE (Ftoroplast);
- Polyvinylidene fluoride (PVDF);
- Intense resins;
- cellulose materials.
Tetrafluoroethane is neutral with respect to many metals. It does not respond with:
- Steel grades 420 AISI (20X13), AISI 431 (14X17H2), AISI 329 (08X21H6M2T), AISI 321 (12X18H10T), AISI 316Ti (10X17H13M2T), AISI 904L (06XH28MDT).
- Nickel and its alloys: N2, KhN78T (EI435), NMZhMts 28-2.5-1, (MONEL alloy 400).
- Aluminum AD1 (AA 1135, 1145, 1230, 1235 and 1345, ENAW-1235, ENAW-Al99).
- Titanium VT1 (ERTi-1, 3.7024, 3.7025, Ti1, Cl1, Ti-P.01, IMI115).
Replacing R12 with R134a
Replacing R-12 with R-134a is a logical step. The production and use of the first is reduced, it becomes more expensive. When changing the refrigerant, consider the following:
- When using r-134a instead of r-12, a complete oil change is required. Refrigerant R12 works on mineral, R134a – on synthetic.
- The cooling capacity of the equipment can decrease or increase by 5-10%.
- The R134a refrigerant molecules are smaller, increasing the likelihood of leakage through joints and seals. The state of the system needs to be monitored. If necessary, replace weak nodes.
- Some synthetic oils contain harsh additives and additives. It is necessary to test their compatibility with sealing materials and system components.
- It is necessary to replace the filter-drier, adsorbent, install an additional one or increase its quantity. Filters designed for R12 will not do the job with R134a.
- When replacing R-12 with R-134a, the expansion valve (expansion valve, thermostat) must be replaced or adjusted. It is also worth calibrating pressure gauges for the appropriate pressure.
P-T comparison trable of the R134a and R12
| P, kPa | t, °C | t, °C | P, kPa | t, °C | t, °C |
|---|---|---|---|---|---|
| R12 | R134a | R12 | R134a | ||
| 25 | -59 | -53 | 600 | 22 | 22 |
| fifty | -45 | -40 | 650 | 25 | 24 |
| 75 | -37 | -35 | 700 | 28 | 27 |
| 100 | -30 | -26 | 750 | 30 | 29 |
| 125 | -24 | -21 | 800 | 33 | 31 |
| 150 | -20 | -17 | 900 | 37 | 36 |
| 175 | -16 | -13 | 1000 | 42 | 39 |
| 200 | -12 | -10 | 1200 | 49 | 46 |
| 225 | -9 | -7 | 1400 | 56 | 52 |
| 250 | -6 | -4 | 1600 | 62 | 58 |
| 275 | -4 | -2 | 1800 | 68 | 66 |
| 300 | -1 | 1 | 2000 | 73 | 67 |
| 325 | 2 | 3 | 2200 | 78 | 72 |
| 350 | 4 | 5 | 2400 | 82 | 76 |
| 375 | 6 | 7 | 2600 | 86 | 79 |
| 400 | 8 | 9 | 2800 | 90 | 83 |
| 450 | 12 | 12 | 3000 | 94 | 86 |
| 500 | 16 | 16 | 3200 | 98 | 89 |
| 550 | 19 | 19 | 3400 | 101 | 93 |
Refrigerant gas r134a and human health
Despite the low toxicity, with careless handling of HFC R134a, side effects of varying severity are possible.
Inhalation
The allowable concentration of refrigerant 134a in air is 0.1% or 1000 ppm. When inhaling the refrigerant with air, such a concentration for 12 hours will not adversely affect human health.
Inhalation of air with a high content of r134a refrigerant can lead to inhibition of the activity of the nervous and cardiovascular systems with side effects:
- Dizziness;
- Headache;
- Decreased mental activity;
- confusion and loss of consciousness;
- violation of coordination;
- Rapid pulse;
- Arrhythmia;
- Pressure drops.
When the concentration of gas in the air is more than 7.5% or 75,000 parts per million, the cardiovascular system becomes receptive to adrenaline. The normal rhythm of the heart is disturbed. In combination with emotional stress and worries, this can lead to a heart attack and death.
Eyes and skin
At room temperature, refrigerant vapor does not affect the eyes and skin. When exposed to R134a in the liquid phase, there is a claim of frostbite. If this happens, it is necessary to wash the damaged areas with warm water and consult a doctor.
Explosiveness
R-134a is not explosive in its normal state. In a mixture with air, it ignites from an open source of fire or high temperature at +100 degrees pi normal atmospheric pressure. The risk of ignition increases with:
- Increasing pressure;
- Increasing the concentration of oxygen in the air;
- Rise in temperature.

Safety
- If there is a risk of liquid refrigerant leakage or splashing, protect eyes and exposed skin;
- When carrying out repairs and maintenance of systems with a large amount of freon r134a, it is necessary to check the premises for leakage with a gas analyzer;
- Technical rooms associated with the operation of cooling systems must be equipped with powerful forced ventilation;
- If a slightly sweet smell appears while working with the refrigerant, immediately leave the room and ventilate it.
- Do not work with r134-a near open flames, heat sources. Do not mix the gas with air or oxygen for any purpose.
- The R134a cylinder cannot be reused. Refueling from isotanks can only be made into new containers or cylinders intended for reusable use.
In this article, we talked about the properties of the R134a refrigerant. We hope you have found the information you need. Don’t forget to share this article with your friends and colleagues!
Хотите получить помощь мастера, специалиста в этой сфере? Переходите на портал поиска мастеров Профи. Это полностью бесплатный сервис, на котором вы найдете профессионала, который решит вашу проблему. Вы не платите за размещение объявления, просмотры, выбор подрядчика.
Если вы сами мастер своего дела, то зарегистрируйтесь на Профи и получайте поток клиентов. Ваша прибыль в одном клике!